Call for Implementation of biomarkers 2025

The long-term programme is meant to translate the most promising biomarker(s) into practice. Biomarker development will be supported until the biomarker is implemented on a national scale. This goal should be achieved within the shortest, yet realistic timeframe possible. KWF funding will be adjusted or ceased when the biomarker reaches a stage where further development should be (partially) taken over by a (for profit) third-party.
To ensure that the ambitious goals will be reached, but also to give room to adapt the project plan, a yearly evaluation and interviews with the project team and its collaborators by an international evaluation committee will form an essential part of the programme. The evaluation committee will provide the project team with feedback and advice and can also give recommendations to adjust the project plan, budget or composition of the project team. It can also determine go/no-go moments and advise KWF to terminate the project prematurely if the ultimate goal is no longer deemed achievable (fail-fast principle). The intention, however, is to make this a success!
To increase the likelihood of success, the project team will be asked to set up a separate expert panel (steering committee) that will provide support and advice on biomarker development. The composition of this panel needs to align with the needs of the specific development phase of the biomarker and may change over time.
To get first insights into medical, social or economic implications of the biomarker, an (early) Health Technology Assessment (HTA) will be part of the evaluation of the full proposals. The HTA will be performed for all full proposals by the same external partner to guarantee uniformity of the analysis. Contact with the external partner and payment of the HTA will be arranged by KWF. In addition, for the runtime of the project, you will be required to have a HTA specialist in the team.
In scope
Projects in which a clinical grade biomarker will be demonstrated in an operational environment (TRL 7). This means that the performance, effectiveness, and reliability of the biomarker is evaluated under conditions that closely mimic everyday clinical practice, including workflow and equipment.
The programme is open to any tumor type. A requirement is that there is a clear clinical need for the biomarker in the oncology field. Preferably, the biomarker can be implemented without increasing treatment costs. Also, the biomarker should only have a single context of use. At the end of the programme, the biomarker needs to be implemented in clinical practice.
The programme is only open for research consortia (4 or more participating parties). Collaboration with for-profit partners is encouraged. It is strongly advised not to put a PhD on the project as projects can be terminated prematurely if the evaluation committee deems this necessary.
There are no time restrictions with respect to runtime of the project. Yet, the evaluation committee will be asked to check whether this is the shortest time frame possible to implement the biomarker. Guideline to nationwide implementation: 5 years if the biomarker is already validated.
KWF is aware that there is more uncertainty when estimating the budget for activities that are far away in the future. Therefore, every year the budget will be re-evaluated and discussed with the evaluation committee. When preparing the budget, start with how you envisage the biomarker being used in clinical practice and then think of all the steps that need to be taken to get there. Do you already foresee a certain point in time where biomarker development is taken over by a for-profit partner?
Eligibility for the long-term programme will be determined using a checklist:
Note: The checklist must be completed and submitted via email to [email protected] prior to submitting the pre-proposal. Without the checklist, the pre-proposal will not be considered. Only projects within scope will be invited to submit a pre-proposal. The rest may be directed to other funding opportunities offered by KWF for biomarker projects (theme call and open call).
Out of scope
- Clinical validation of research grade biomarkers
- Funding of a whole biomarker research line
- Biomarker projects below TRL7
- Biomarkers which predict side effects of treatment
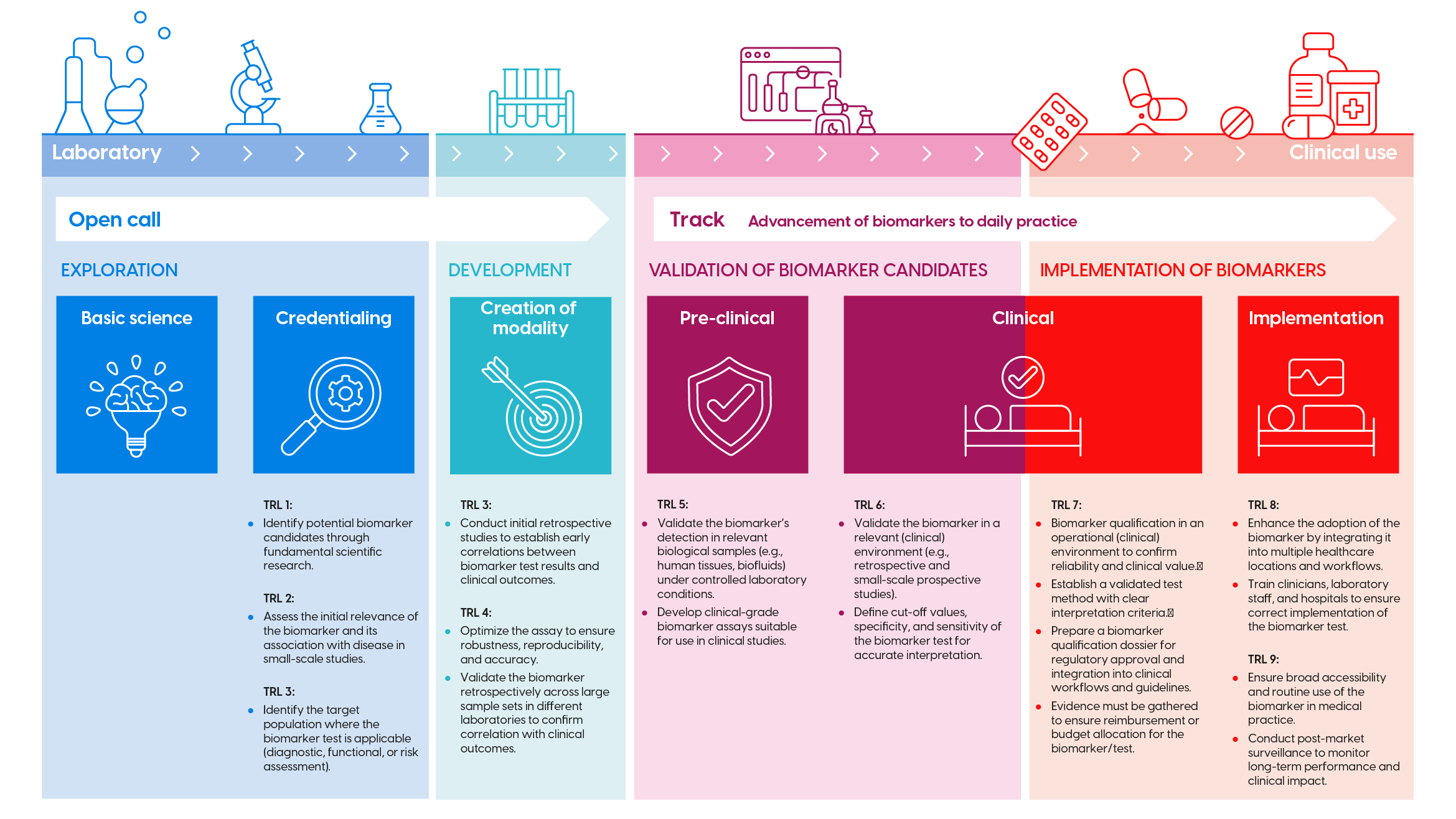
Biomarker development trajectory (click image to enlarge)
The development of biomarkers consists of successive research phases and Technology Readiness Levels (TRL). These are visualized in the infographic above (click to enlarge). For each phase, KWF has corresponding funding opportunities (open calls, theme call, etc.). Check the infographic to determine if your proposal fits the long-term programme.
Terms & conditions
The KWF Funding Conditions 2022 and the KWF Accountants Protocol 2022 apply (see downloads).
In addition, the following conditions apply:
- An HTA expert is part of the project team.
- The budget for additional expertise that does not fall within the NFU salary scales can be requested in GMS as a 'service provider'. KWF applies maximum hourly rates for the hiring of freelancers (external hiring): supportive (MBO): €85; project management (HBO): €100, specialist (WO): €125. These are the maximum hourly rates excluding VAT and including all other costs (travel expenses, parking fees, travel time, etc.). For external service providers, quotes must be submitted specifying the effort in hours. For hiring external expertise where these maximum hourly rates are exceeded, multiple quotes from different parties must be submitted along with a justification of why this party is the most suitable; the justification and quotes will be evaluated by the evaluation committee.
- A letter of intent/support is mandatory when a for-profit partner is involved in the project.
- If a for-profit partner (>250 FTE) has an active role within the project, this is considered a participating party and therefore must make a (financial) contribution: this is 10% co-financing (in kind and/or in cash) of the budget requested by KWF.
- Before the start of the project, a steering committee approved by KWF must be established.
- Twice a year, a meeting will take place involving the project team, the steering committee, and KWF. The main task of the steering committee is to advise the project leader with the aim of maximizing the chance of successful implementation.
- Every year, the progress of the project will be evaluated by the evaluation committee. This includes whether milestones and go/no-go moments have been reached, but also project plans and whether the composition of the team fits the current development phase of the biomarker. The evaluation committee also evaluates the budget. A negative evaluation can lead to termination of the project.
Timeline
| Opening checklist: | 3 September 2024 |
| Opening pre-proposals: | 24 September 2024 |
| Closure checklist: | 22 October 2024 |
| Closure pre-proposals: | 3 December 2024 (12.00 noon) |
| Opening full proposals: | 28 January 2025 |
| Closure full proposals: | 1 April 2025 (12.00 noon) |
| Interviews: | 27 May 2025 |
| Funding decision: | July 2025 |
Indicative budget and duration
Budget per proposal: 1-4 million
Duration: 3-5 years